Transition Metal Complexes as Oxidation Catalysts
-
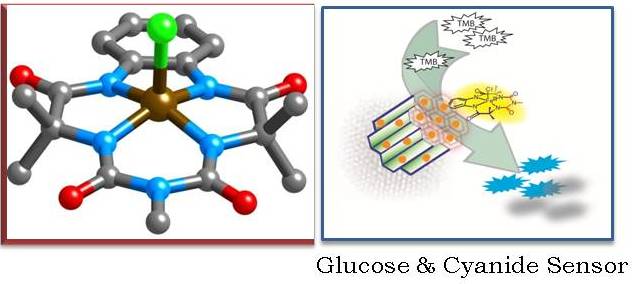
Designing metal complexes that are functional mimic of metalloenzymes which activate H2O2 to perform selective oxidation but are themselves inert to oxidation is the key to the synthesis of efficient transition metal oxidation catalysts. In recent years, several metal complexes that activate H2O2 have been synthesized from biologically relevant transition metals and a myriad of electron donating ligands. However, as catalysts for oxidation of organic compounds, all of them fall short in terms of selectivity and turnover numbers in comparison to the metalloenzymes. This has limited their application in both organic synthesis and in analytical biochemistry. We develop Fe complexes of macrocyclic biuret ligands that would perform selective oxidation with turnover numbers comparable to the metalloenzymes. Such complexes have application as catalysts for oxidation of unactivated C-H bonds and as chemical probes for signal amplification in the detection of analytes.
-
Polypeptide based Nanomaterials
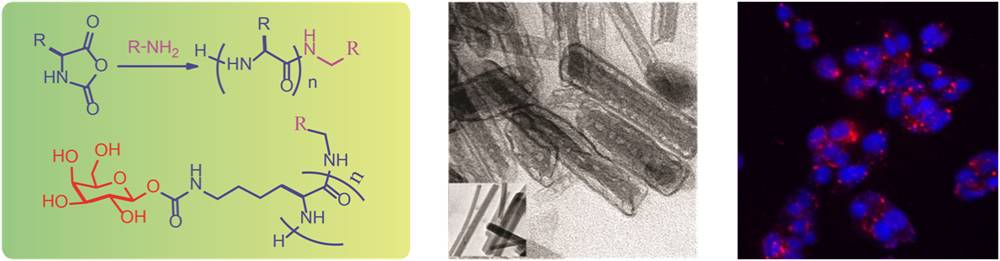
- Synthetic polypeptides, polymers made from amino acids, have found wide applications in biotechnology such as in tissue engineering and drug delivery. Polypeptide polymers have many advantages over synthetic polymers because they are biocompatible and also possess stable secondary structure (α-helix and β-sheet) that are commonly found in proteins. These secondary structures also strongly contribute to the self-assembling character of the polypeptide chains that gives rise to three dimensional nanostructures from these linear macromolecules. In addition to this, the presence of a wide range of side chains (such as amines, thiols and carboxylic acid) in the polypeptides make them responsive to external stimuli such as pH, temperature, solvent and electrolytes. These polypeptides are synthesized in our laboratory by the polymerization of α-amino acid-N-carboxyanhydrides (NCA). We have synthesized several polypeptides having both natural and non-natural amino acid side chains. For example, glycopolypeptides (glycopolymers with pendant carbohydrates on a polypeptide backbone) have been synthesized in our laboratory. We also study the self-assembly of these polypeptides into nanostructures such as micelles, vesicles, nano-rods and their biomedical application in drug delivery, tissue engineering and gene transfection.
Collaborators: Prof. S. Hotha (IISER-Pune) and Dr. A. Ambade (NCL)
-
Porous Materials
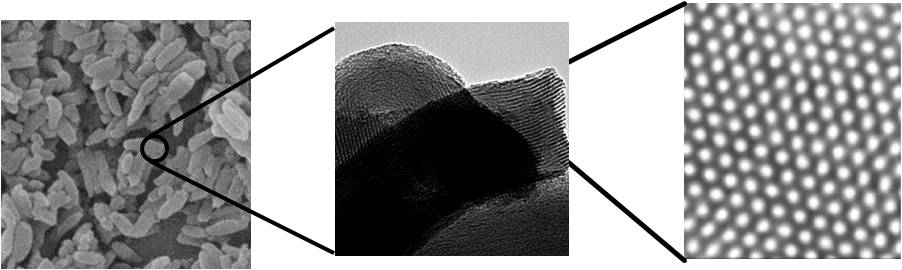
- Mesoporous materials display a honeycomb-like structure of uniform mesopores (3-15 nm in diameter) running through a matrix of amorphous silica. Their high porosity and concomitant large surface area make them ideally suited ideally suited to be various applications in chemistry and biology. We synthesize hydrid materials that combine the properties of organic and inorganic building blocks within a single material. This is particularly attractive from the viewpoint of materials scientists because of the possibility to combine the enormous functional variation of organic chemistry with the advantages of a thermally stable and robust inorganic substrate. We have developed organo-azide functionalized SBA-15 and MCM-41 mesoporous materials that can be used to attach various organic molecules, inorganic complexes, nanoparticles and polymers onto their surface by “click chemistry”. These hybrid materials are being used for applications in catalysis, drug delivery and sensor design.
- Figure: SEM and TEM images of SBA-15 displaying the porous structure having hexagonal channels
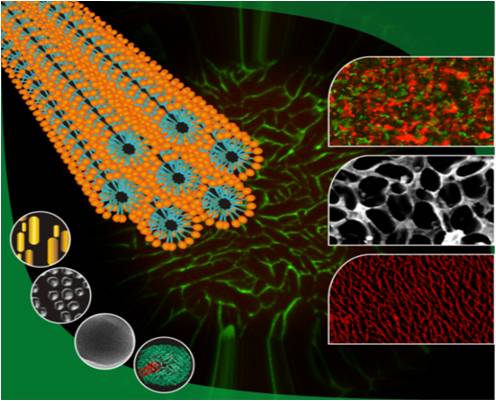
- We also work on the assembly of nanoparticles into free-standing macroporous materials by the dynamic templating of surfactant hexagonal domains that was developed by Dr Guruswamy’s group at NCL. These materials have a wide range of applications in separation, chromatography, catalysis, and as biomedical scaffolds. Currently, we are trying to develop porous biocompatible scaffolds that can be used as an extracellular matrix for soft tissue engineering.
Collaborators: Dr. K. Guruswamy (NCL); Prof Giyoong Tae (GIST, Korea)
Collaborators:
- Dr. K. Guruswamy (NCL)
- Dr. BLV Prasad (NCL)
-
|