|
Our research focuses on the discovery, development, and mechanistic study of new chemical reactions, guided by the challenges and opportunities presented in natural product total synthesis. Natural products have long served as both valuable therapeutic agents and vital inspirations for innovation in synthetic organic chemistry. Their intricate architectures, dense functionality, and stereochemical complexity create an ideal testing ground for reaction development, demanding high levels of selectivity and efficiency. By pursuing total synthesis, we aim to push the boundaries of modern organic chemistry while contributing broadly to fields that rely on advanced synthetic methods, including catalysis, medicinal chemistry, and chemical biology. Total Synthesis of Biologically Active Natural Products Total synthesis of biologically active natural products is vital for understanding complex molecular structures, enabling structure-activity relationship studies, and facilitating drug development. It allows access to scarce or unavailable natural compounds, supports the discovery of novel therapeutics, and drives innovation in synthetic methodology, contributing significantly to medicinal and organic chemistry.
Development of Novel Methodologies Spirocyclic natural products, with diverse ring systems and all-carbon quaternary centers, are key pharmacophores valued in drug discovery. Their synthesis is challenging, especially spirocarbocycles, prompting ongoing efforts. Notably, 1,4-diketospiranes and cyclic 1,4-keto esters remain underexplored, with HAT-initiated Dowd–Beckwith rearrangement offering a promising strategy for their construction.
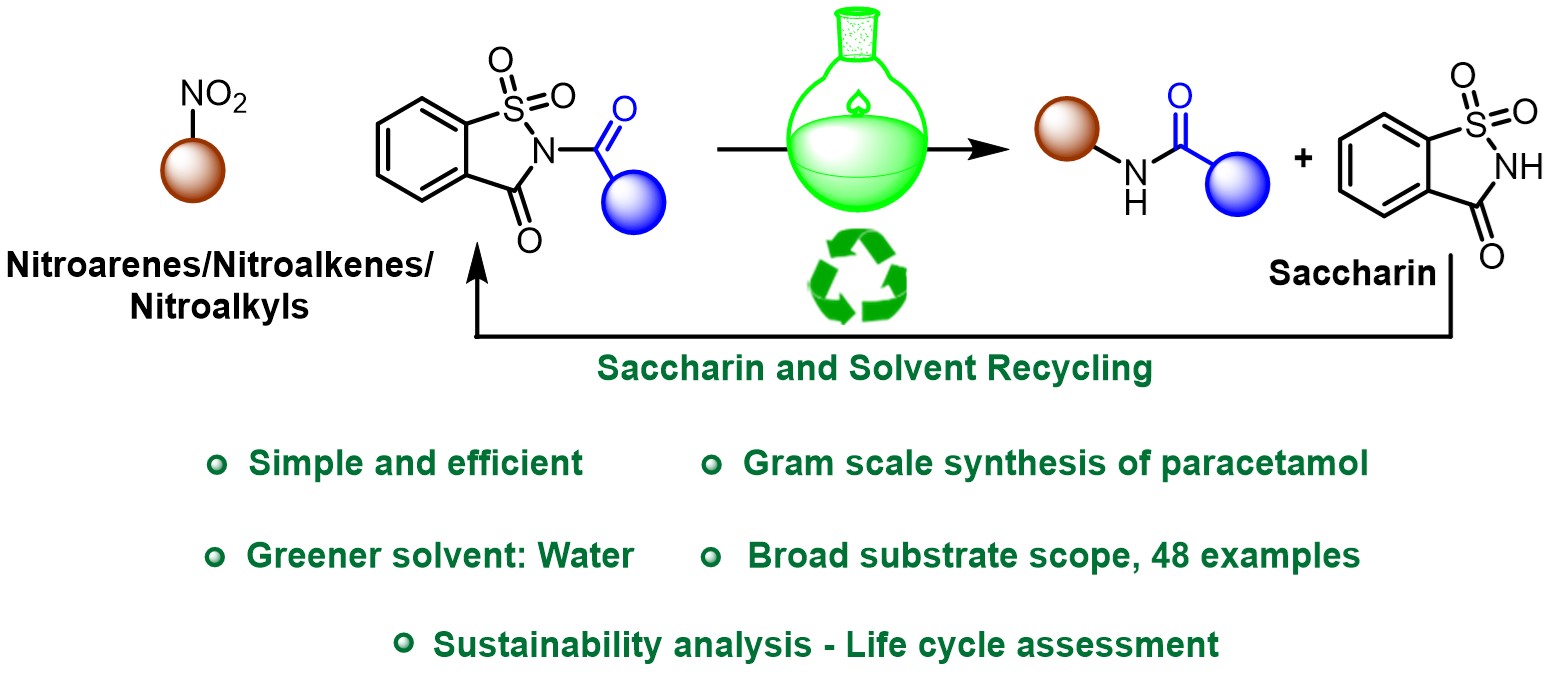
Green and Sustainable Chemistry Amide bonds are essential in pharmaceuticals and materials, yet current syntheses often rely on toxic reagents and solvents. Green, scalable, and water-based alternatives are urgently needed for sustainable development. We developed a green method to form N-substituted amides from nitroarenes using stable, affordable N-acyl saccharins as acyl donors in water.
|