|
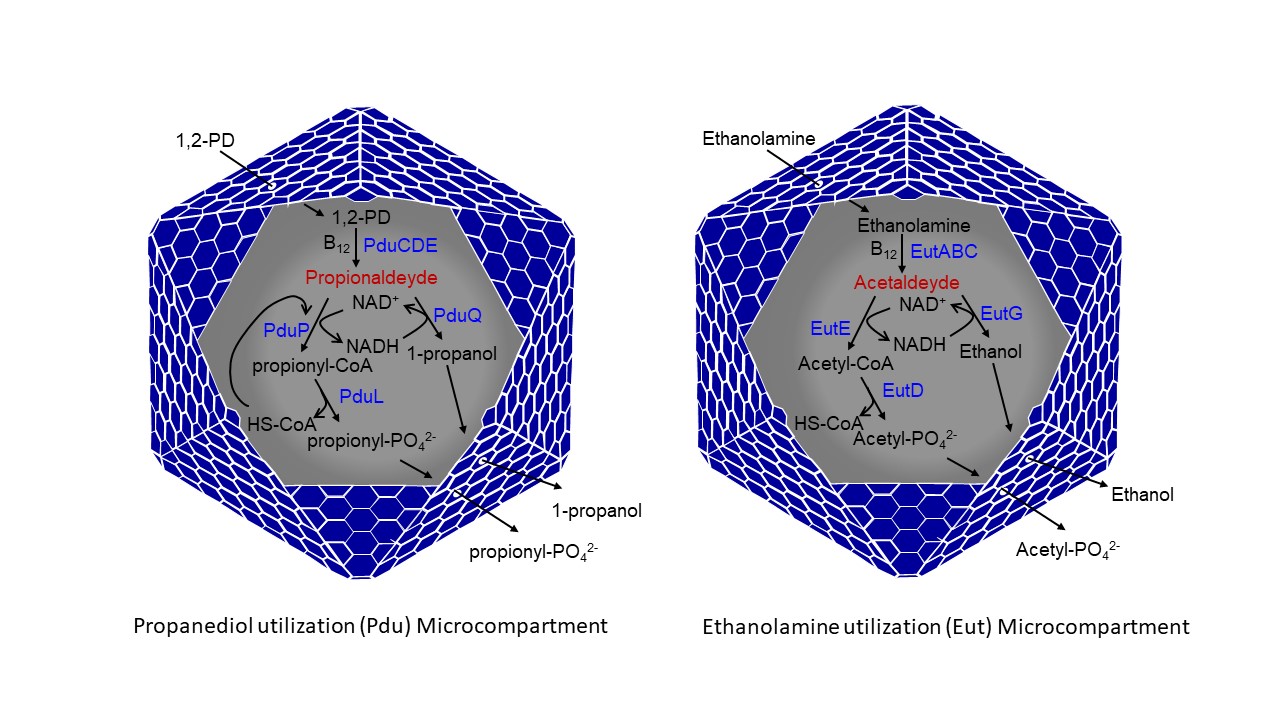
Project 1: Bacterial Microcompartments and their role in modulating gut microbiome physiology Bacterial microcompartments (MCPs) are sophisticated protein-based organelles that encapsulate enzymes involved in specific metabolic pathways. The genomic presence of MCPs and their expression have been linked to bacterial pathogenesis in several enteric bacteria including Salmonella, Escherichia, Klebsiella, Pseudomonas and Listeria. These pathogens maintain operons that encode proteins enabling them to form large protein complexes. These protein structures serve to encapsulate metabolic enzymes that permit microbial growth and survival on substrates such as 1,2-propanediol, ethanolamine or choline in the hostile environment of human gut. Studies indicate that metabolism of the above substrates inside MCPs provides enteric pathogens a competitive growth advantage over other intestinal micro flora. Chowdhury lab is interested to understand metabolisms occurring in MCPs in terms of catabolic and structural components of the pathway, its regulatory mechanisms and its potential roles in pathogenesis, which would perhaps provide insights into operational principles of bacterial MCPs with the development of new methods of pathogen control.
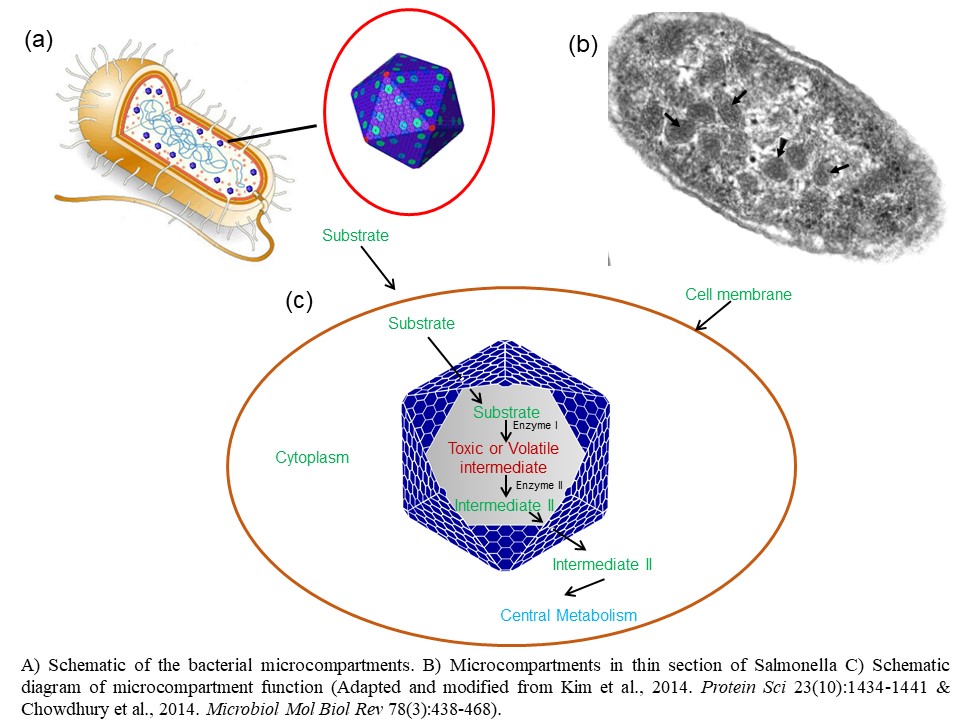
Project 2: Utilization of bacterial microcompartment in biotechnology applications The optimization of biological processes through compartmentalization is a strategy found throughout the biological world. On the cellular level, organelles act as compartments that allow spatial and temporal control of biochemical reactions, as well as provide controlled microenvironments for these reactions. Bacteria lack the traditional membrane enclosed organelles associated with eukaryotes. However, recent studies found that many bacterial species utilize protein-based microcompartments (MCPs) as organelles for the optimization of metabolic processes. Unlike eukaryotic organelles bacterial MCPs have polyhedral protein shells that structurally resemble viral capsids, though there is no evidence for evolutionary relationship between the two structures. The MCP shell, roughly 80-200 nm in diameter, is entirely made of proteins and is composed of thousands of copies of protein subunits.
Bacterial MCPs enhance reaction rates by creating high local concentrations of enzymes and substrates, confinement of pathway intermediates that are toxic or readily lost by evaporation, and use of private cofactor pools as well as minimization of side reactions. In addition, MCPs are self-assembling, selectively permeable and are a promising basis for engineering compartmentalized pathways. Therefore, bacterial microcompartments have great promise to serve as molecular chambers in terms of cargo delivery in food and pharmaceutical industries. Chowdhury lab is interested to utilize MCPs as design templates for the engineering of subcellular nanobioreactors that can be customized to support new metabolic functions.
Google Scholar id: scholar.google.co.in/citations
Orcid id: orcid.org/0000-0003-2026-5324 |